What is salinity stress in plants? How it impacts the soil
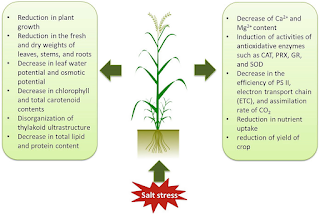
Around the world, soil salinization hinders crop growth and yield due to its environmental effects. The effects of salinity stress in plants range from physiological to biochemical. Reduced water potential in the soil solution, ionic disequilibrium, specific ion effects, and a rise in reactive oxygen species (ROS) are all consequences of its presence. Phytohormones, osmoprotectants, antioxidants, polyamines, and trace elements have all been investigated in recent years to minimize damages caused by salinity stress. There is evidence that selenium (Se), one of the protective substances, can improve plant growth and increase tolerance to excessive soil salinity.
There is, however, still a need for more in-depth studies of the mechanisms of Se-induced salinity tolerance. Reviewing the knowledge involved in Se-mediated improvements of plant growth after exposure to salinity, this review offers future perspectives as well as some limitations on future research in this field.
1. How salinity affects plant growth
The problem of salinity stress in plants arises when sufficient ions accumulate in the root zone to negatively affect plant growth. Plant roots cannot withdraw water from surrounding soil when salts are present in the root zone. Regardless of the amount of water in the root zone, this decreases the amount of water available to the plant. Plants are able to use more water from the soil with salt-free water when compared to two identical soils with the same moisture levels. When plants grow in soils with salty water versus salt-free water, plants use more water from the soil with salt-free water. Despite the fact that the water in saline environments doesn't hold tighter to the soil, the presence of salt in the water causes plants to exert more energy in order to extract water from the soil. Plants can suffer from stress when soil water is excessively salinized.
Salinity in soil water is influenced by soil type, climate, water use, and irrigation practices. Soil available water increases immediately after irrigating the soil, and soil salinity decrease. In order to conserve soil water, plants use more of the remaining water, which gets progressively harder to get. During transpiration or evaporation, plants take up water and evaporation removes it from the soil, thus increasing the salinity of the remaining soil water. Salinity can be further increased by evapotranspiration (ET) between irrigation periods.
2. Physical properties of soil affected by salinity
Fine particles can bind together into aggregates as a result of soil water salinity. As a result, roots penetrate the soil more deeply, and growth is stimulated. This is known as flocculation.
The presence of salt in soil solution can help soil aggregate and stabilize, but at high salinities, the presence of salt can be detrimental to plants. To maintain soil structure, salinity cannot be increased without considering the effects on plant health.
3. Physical properties of soil affected by sodium and sodicity
Salinity stress in plants has the opposite effect on soil as sodium does. The swelling of clay platelets and aggregates are two primary physical processes associated with high sodium concentrations. If too many sodium ions are present between clay particles, the forces that hold them together are disrupted. During the separation process, clay particles expand, causing swelling and soil dispersion.
Clay particles disperse in the soil and block soil pores, reducing soil permeability. Wet and dry soils reform and solidify into almost cement-like soils when the clay is dispersed and you repeatedly wet and dry them. Sodium-induced dispersion is responsible for three major problems: reduced infiltration reduced hydraulic conductivity, and crusting on the surface.
Salinity-producing salts, such as calcium and magnesium, do not have this effect because they tend to cluster closer to clay particles. Because calcium and magnesium compete with sodium for the same spaces to bind to clay particles, they tend to keep soil flocculated. The amount of sodium-induced dispersion can be reduced by increased calcium and magnesium levels.



Comments
Post a Comment